With a new molecule-based method, physicists peer inside an atom’s nucleus
Physicists at MIT have developed a new way to probe inside an atom’s nucleus, using the atom’s own electrons as “messengers” within a molecule.
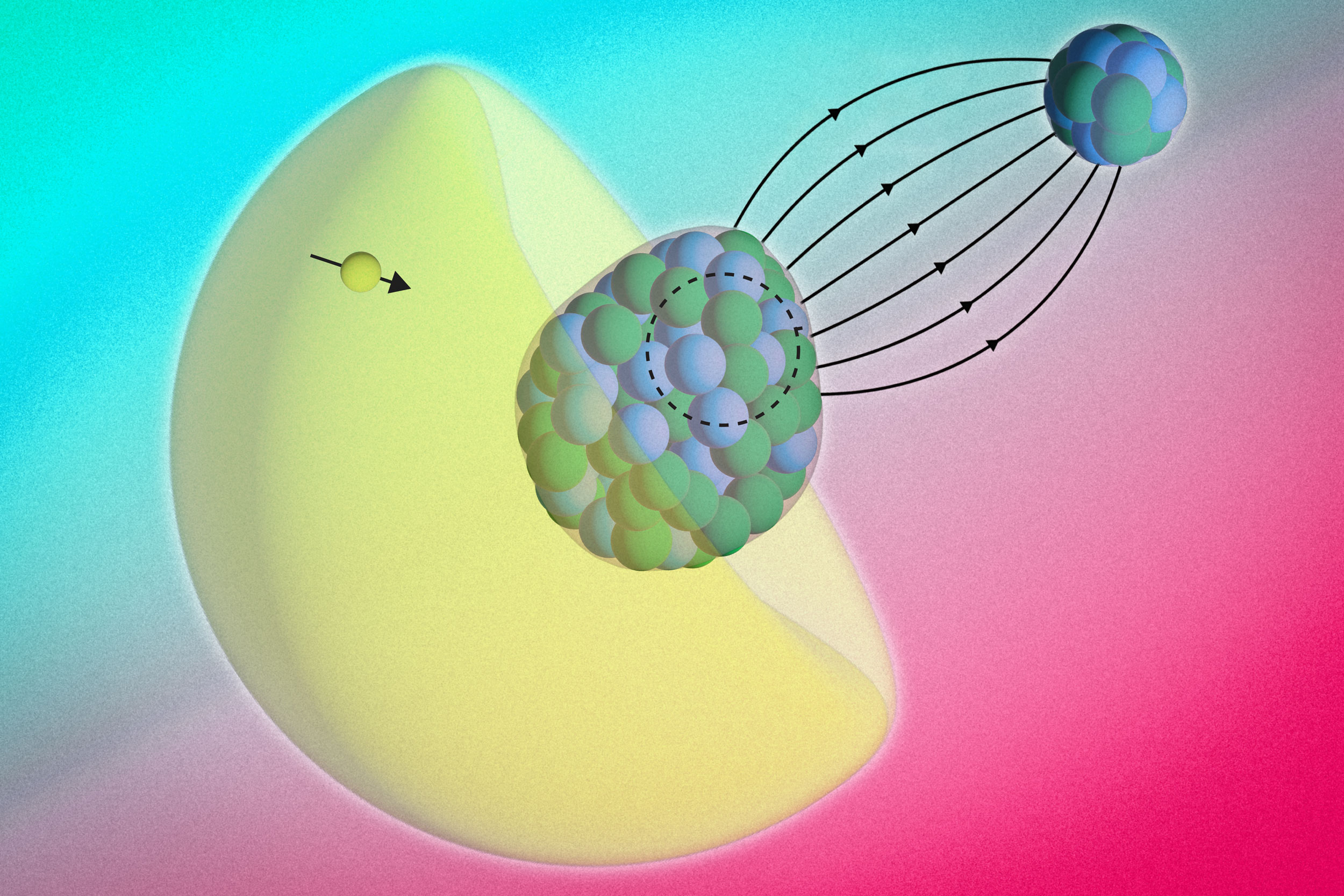
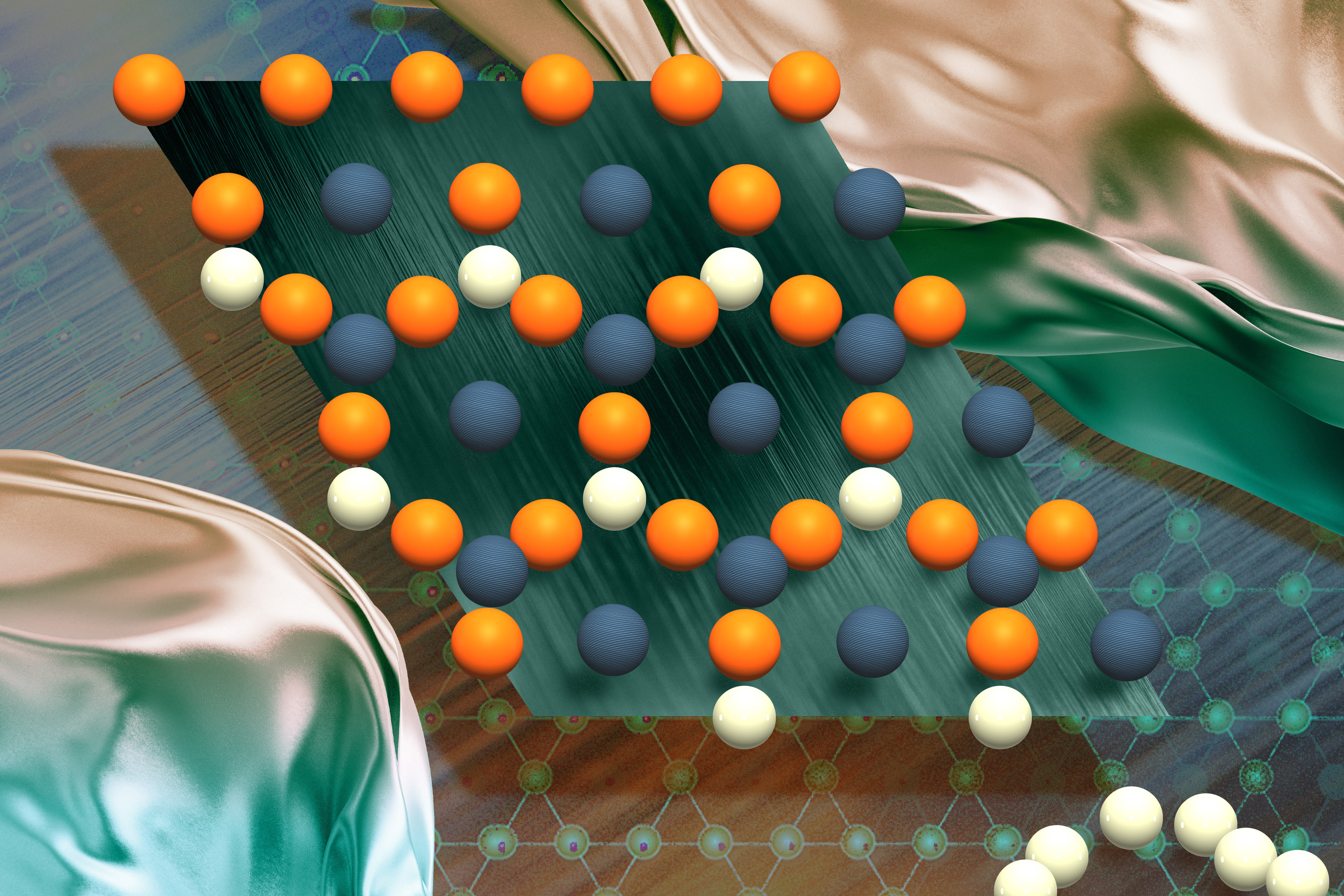
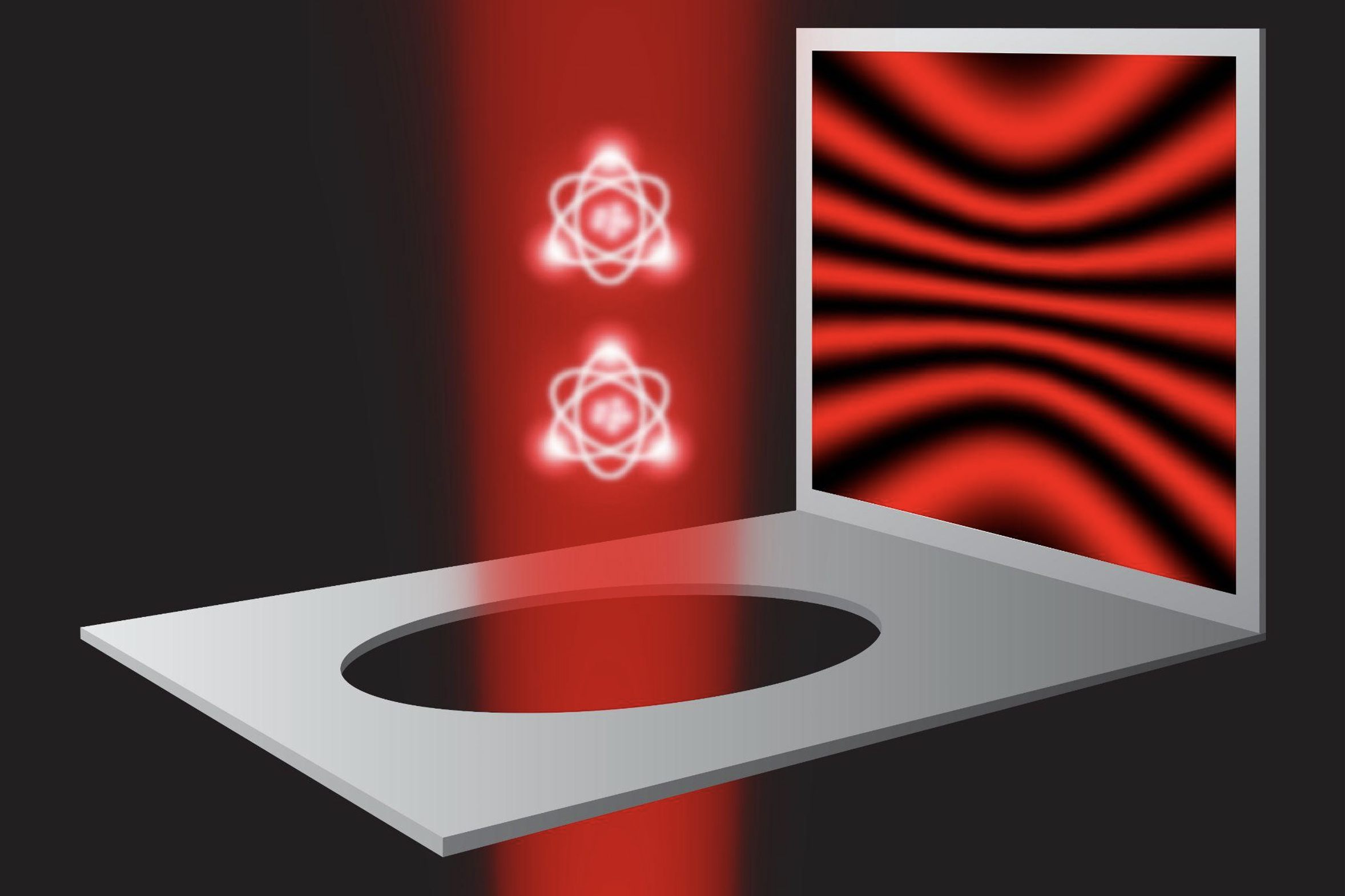
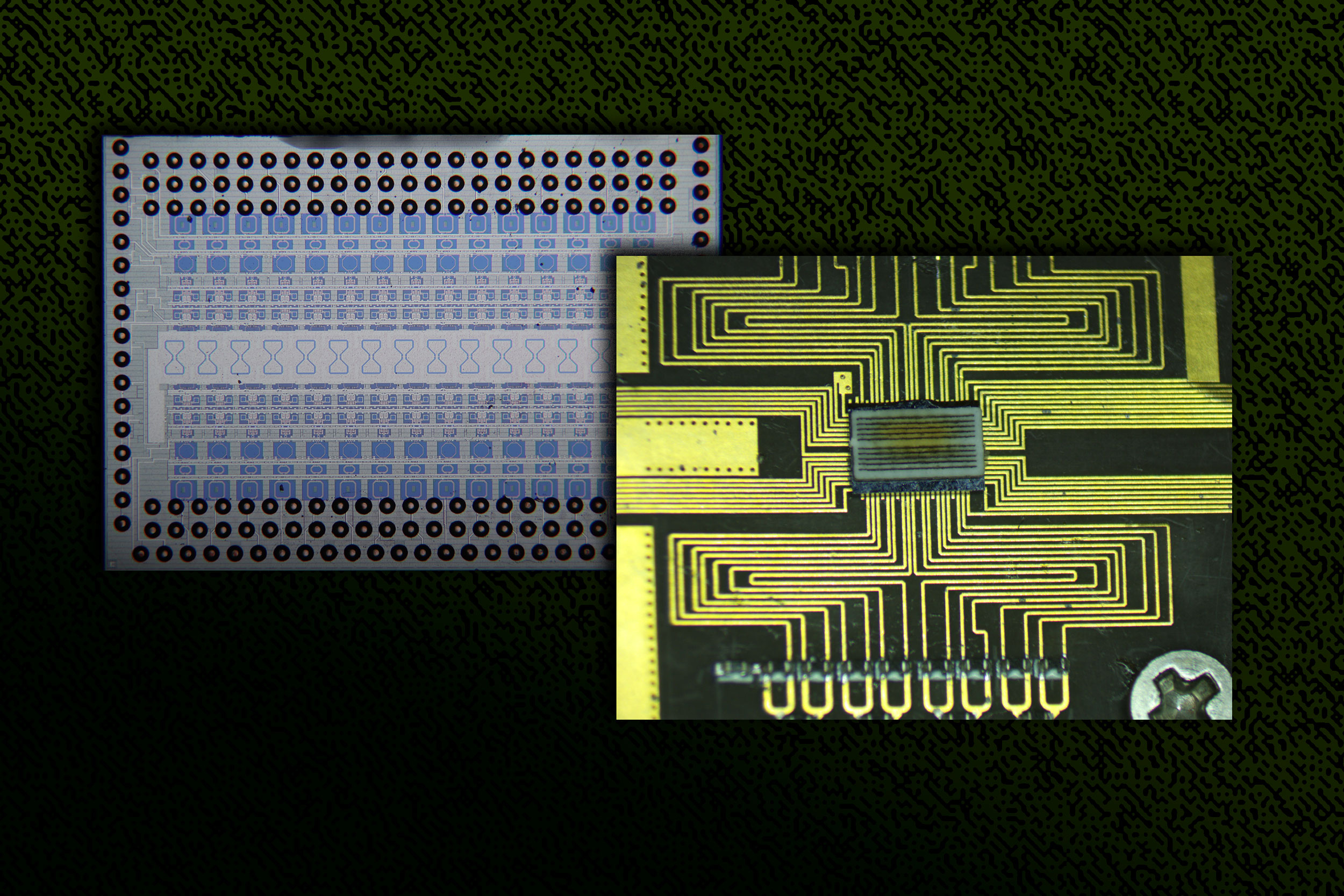
In a study appearing today in the journal Science, the physicists precisely measured the energy of electrons whizzing around a radium atom that had been paired with a fluoride atom to make a molecule of radium monofluoride. They used the environments within molecules as a sort of microscopic particle collider, which contained the radium atom’s electrons and encouraged them to briefly penetrate the atom’s nucleus.
Typically, experiments to probe the inside of atomic nuclei involve massive, kilometers-long facilities that accelerate beams of electrons to speeds fast enough to collide with and break apart nuclei. The team’s new molecule-based method offers a table-top alternative to directly probe the inside of an atom’s nucleus.
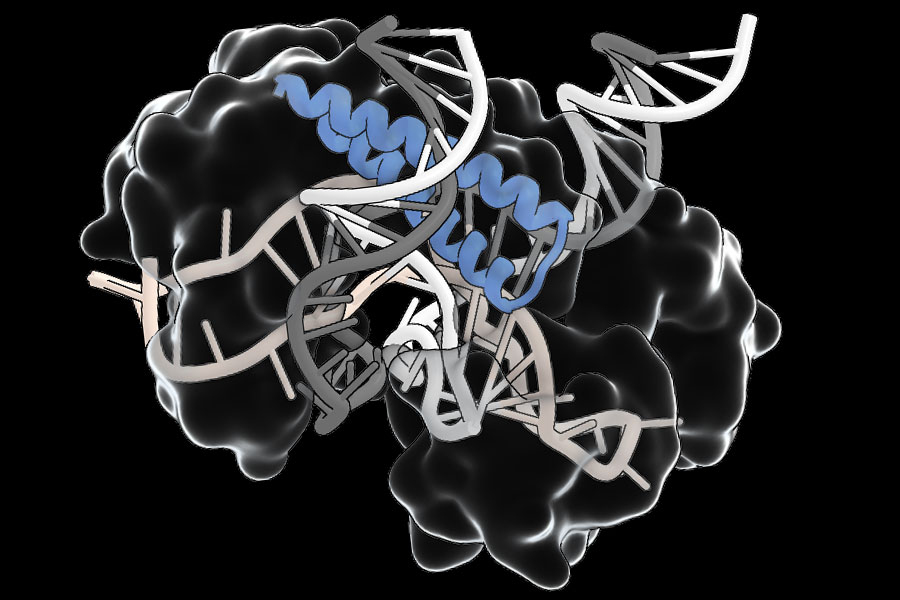
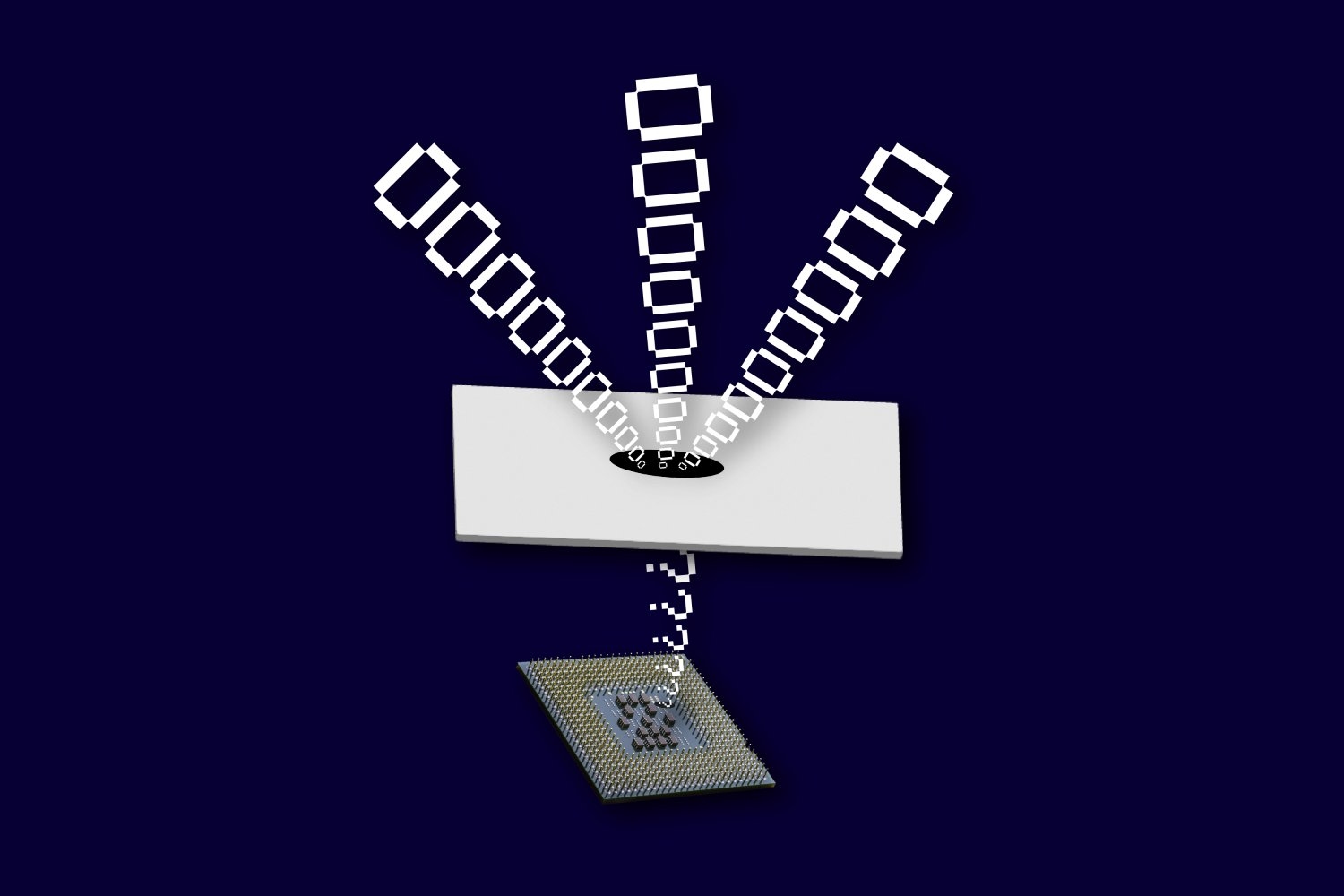
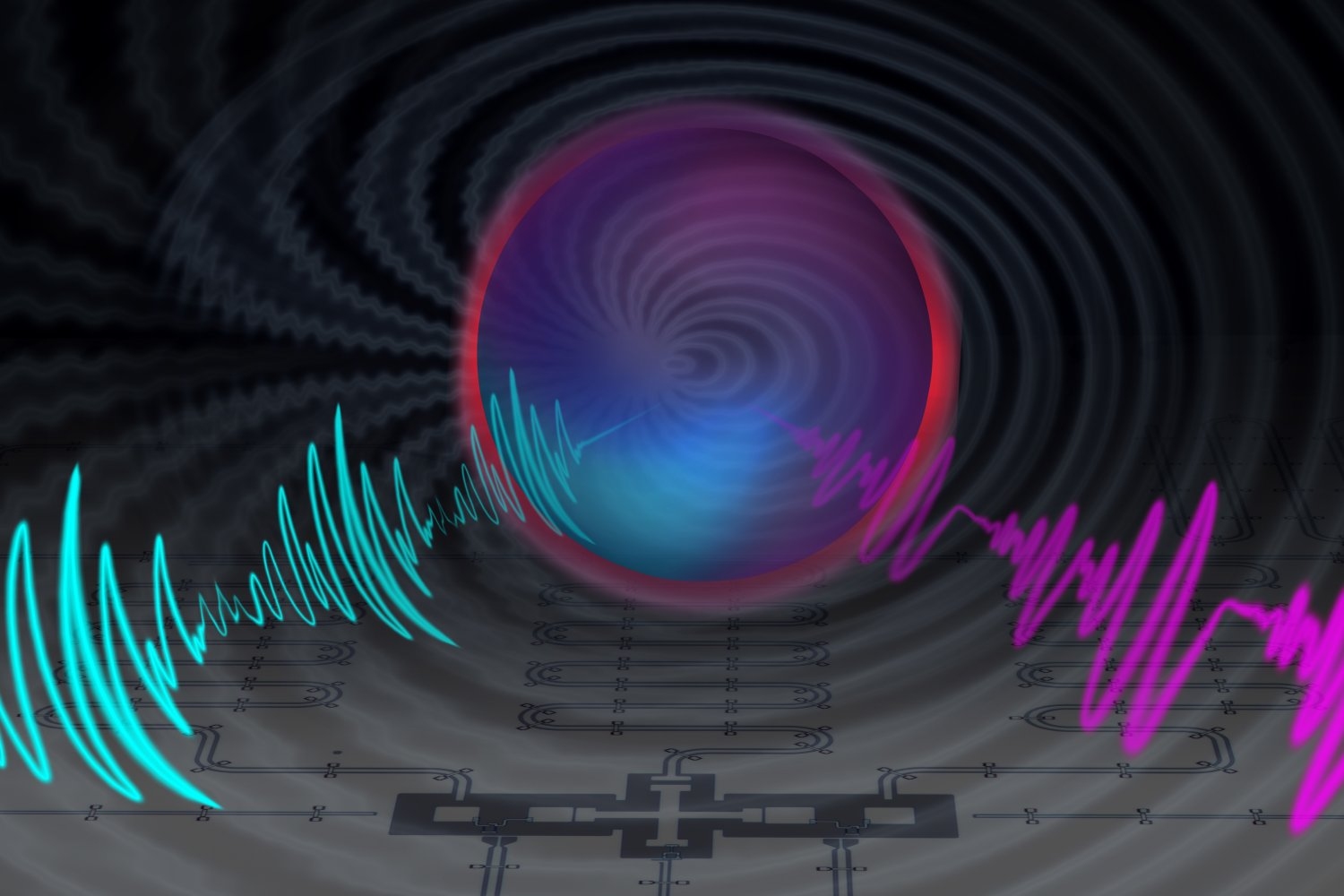
Within molecules of radium monofluoride, the team measured the energies of a radium atom’s electrons as they pinged around inside the molecule. They discerned a slight energy shift and determined that electrons must have briefly penetrated the radium atom’s nucleus and interacted with its contents. As the electrons winged back out, they retained this energy shift, providing a nuclear “message” that could be analyzed to sense the internal structure of the atom’s nucleus.
The team’s method offers a new way to measure the nuclear “magnetic distribution.” In a nucleus, each proton and neutron acts like a small magnet, and they align differently depending on how the nucleus’ protons and neutrons are spread out. The team plans to apply their method to precisely map this property of the radium nucleus for the first time. What they find could help to answer one of the biggest mysteries in cosmology: Why do we see much more matter than antimatter in the universe?
“Our results lay the groundwork for subsequent studies aiming to measure violations of fundamental symmetries at the nuclear level,” says study co-author Ronald Fernando Garcia Ruiz, who is the Thomas A. Franck Associate Professor of Physics at MIT. “This could provide answers to some of the most pressing questions in modern physics.”
The study’s MIT co-authors include Shane Wilkins, Silviu-Marian Udrescu, and Alex Brinson, along with collaborators from multiple institutions including the Collinear Resonance Ionization Spectroscopy Experiment (CRIS) at CERN in Switzerland, where the experiments were performed.
Molecular trap
According to scientists’ best understanding, there must have been almost equal amounts of matter and antimatter when the universe first came into existence. However, the overwhelming majority of what scientists can measure and observe in the universe is made from matter, whose building blocks are the protons and neutrons within atomic nuclei.
This observation is in stark contrast to what our best theory of nature, the Standard Model, predicts, and it is thought that additional sources of fundamental symmetry violation are required to explain the almost complete absence of antimatter in our universe. Such violations could be seen within the nuclei of certain atoms such as radium.
Unlike most atomic nuclei, which are spherical in shape, the radium atom’s nucleus has a more asymmetrical configuration, similar to a pear. Scientists predict that this pear shape could significantly enhance their ability to sense the violation of fundamental symmetries, to the extent that they may be potentially observable.
“The radium nucleus is predicted to be an amplifier of this symmetry breaking, because its nucleus is asymmetric in charge and mass, which is quite unusual,” says Garcia Ruiz, whose group has focused on developing methods to probe radium nuclei for signs of fundamental symmetry violation.
Peering inside the nucleus of a radium atom to investigate fundamental symmetries is an incredibly tricky exercise.
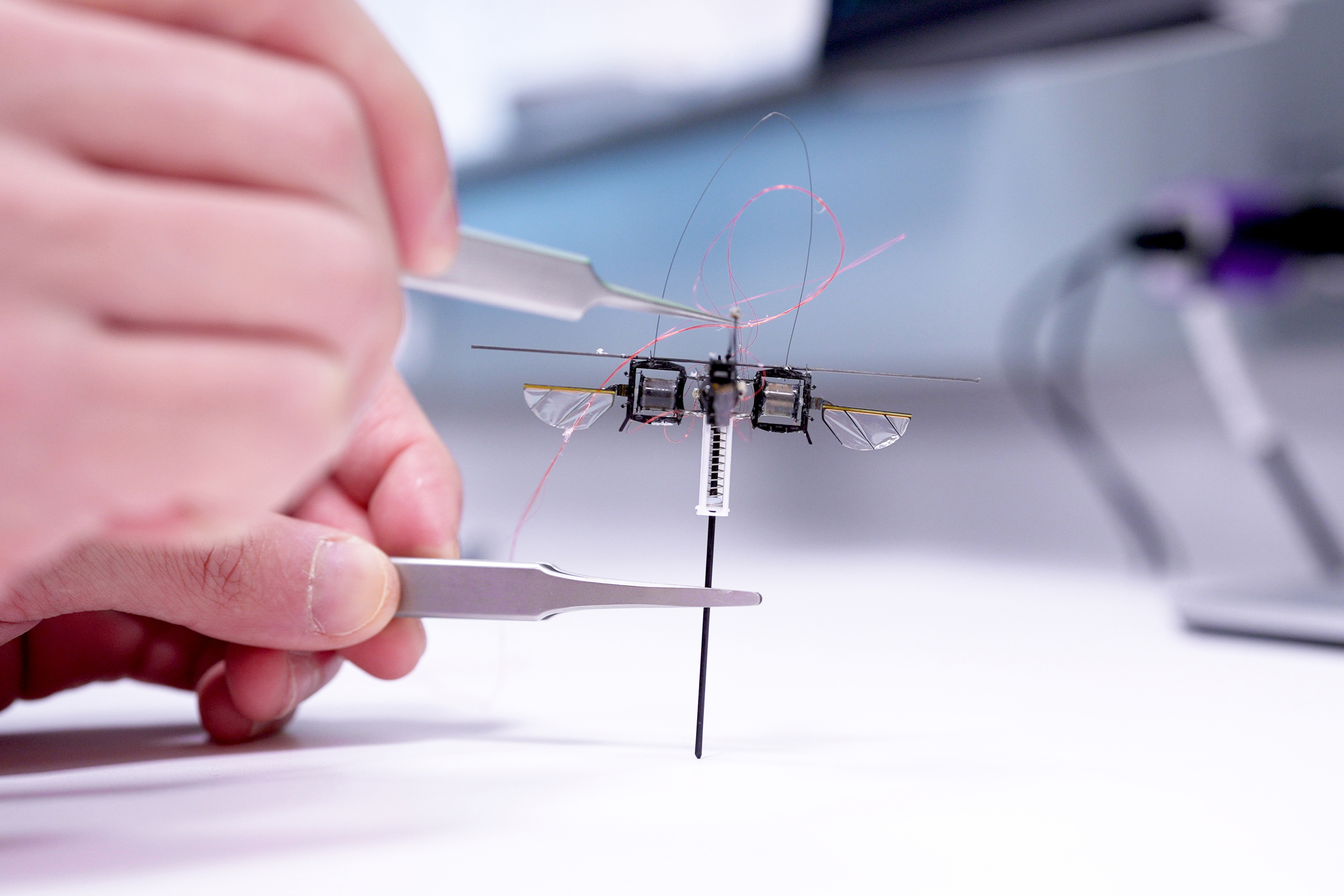
“Radium is naturally radioactive, with a short lifetime and we can currently only produce radium monofluoride molecules in tiny quantities,” says study lead author Shane Wilkins, a former postdoc at MIT. “We therefore need incredibly sensitive techniques to be able measure them.”
The team realized that by placing a radium atom in a molecule, they could contain and amplify the behavior of its electrons.
“When you put this radioactive atom inside of a molecule, the internal electric field that its electrons experience is orders of magnitude larger compared to the fields we can produce and apply in a lab,” explains Silviu-Marian Udrescu PhD ’24, a study co-author. “In a way, the molecule acts like a giant particle collider and gives us a better chance to probe the radium’s nucleus.”
Energy shift
In their new study, the team first paired radium atoms with fluoride atoms to create molecules of radium monofluoride. They found that in this molecule, the radium atom’s electrons were effectively squeezed, increasing the chance for electrons to interact with and briefly penetrate the radium nucleus.
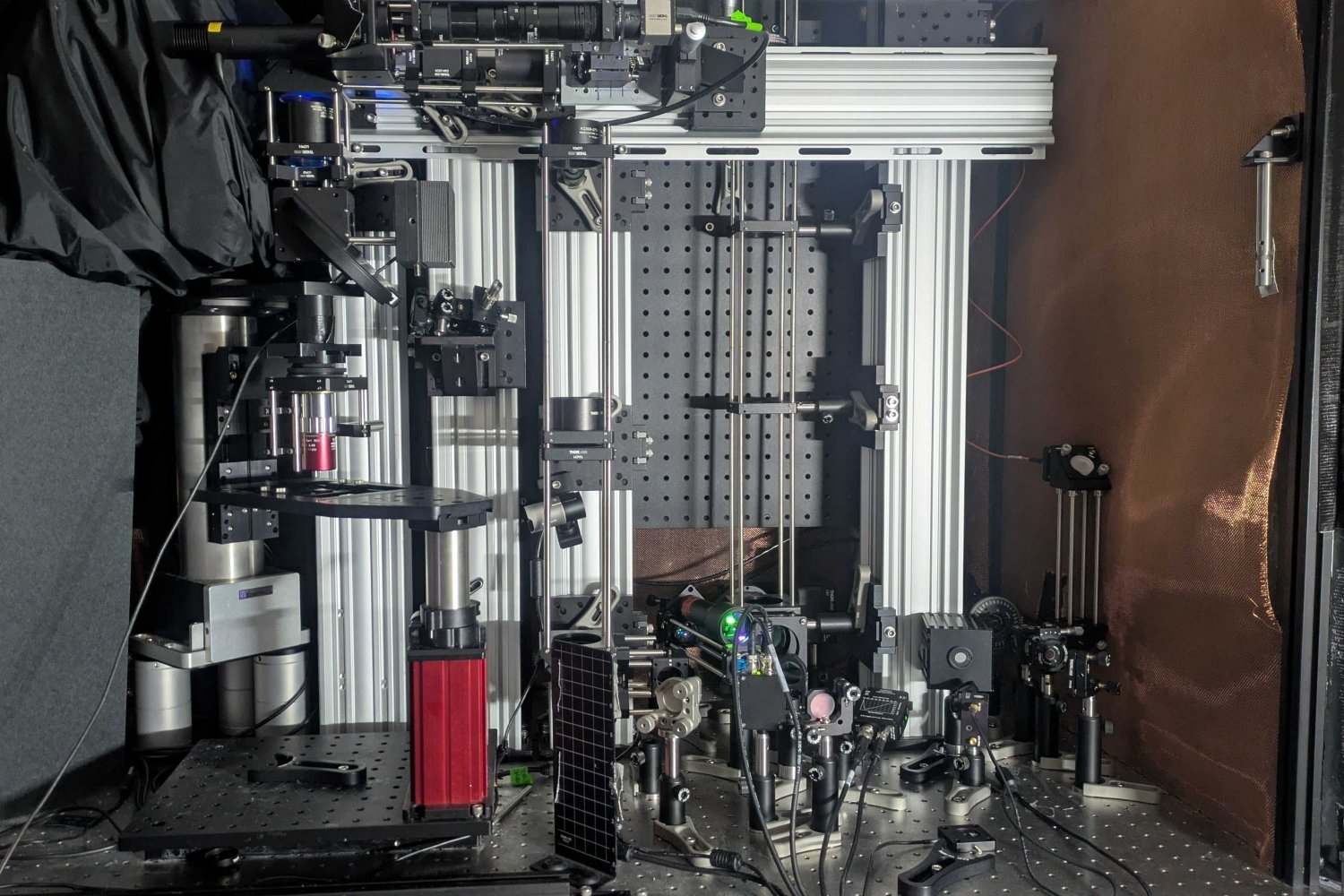
The team then trapped and cooled the molecules and sent them through a system of vacuum chambers, into which they also sent lasers, which interacted with the molecules. In this way the researchers were able to precisely measure the energies of electrons inside each molecule.
When they tallied the energies, they found that the electrons appeared to have a slightly different energy compared to what physicists expect if they did not penetrate the nucleus. Although this energy shift was small — just a millionth of the energy of the laser photon used to excite the molecules — it gave unambiguous evidence of the molecules’ electrons interacting with the protons and neutrons inside the radium nucleus.
“There are many experiments measuring interactions between nuclei and electrons outside the nucleus, and we know what those interactions look like,” Wilkins explains. “When we went to measure these electron energies very precisely, it didn’t quite add up to what we expected assuming they interacted only outside of the nucleus. That told us the difference must be due to electron interactions inside the nucleus.”
“We now have proof that we can sample inside the nucleus,” Garcia Ruiz says. “It’s like being able to measure a battery’s electric field. People can measure its field outside, but to measure inside the battery is far more challenging. And that’s what we can do now.”
Going forward, the team plans to apply the new technique to map the distribution of forces inside the nucleus. Their experiments have so far involved radium nuclei that sit in random orientations inside each molecule at high temperature. Garcia Ruiz and his collaborators would like to be able to cool these molecules and control the orientations of their pear-shaped nuclei such that they can precisely map their contents and hunt for the violation of fundamental symmetries.
“Radium-containing molecules are predicted to be exceptionally sensitive systems in which to search for violations of the fundamental symmetries of nature,” Garcia Ruiz says. “We now have a way to carry out that search.”
This research was supported, in part, by the U.S. Department of Energy.

© Image: Courtesy of the researchers; edited by MIT News